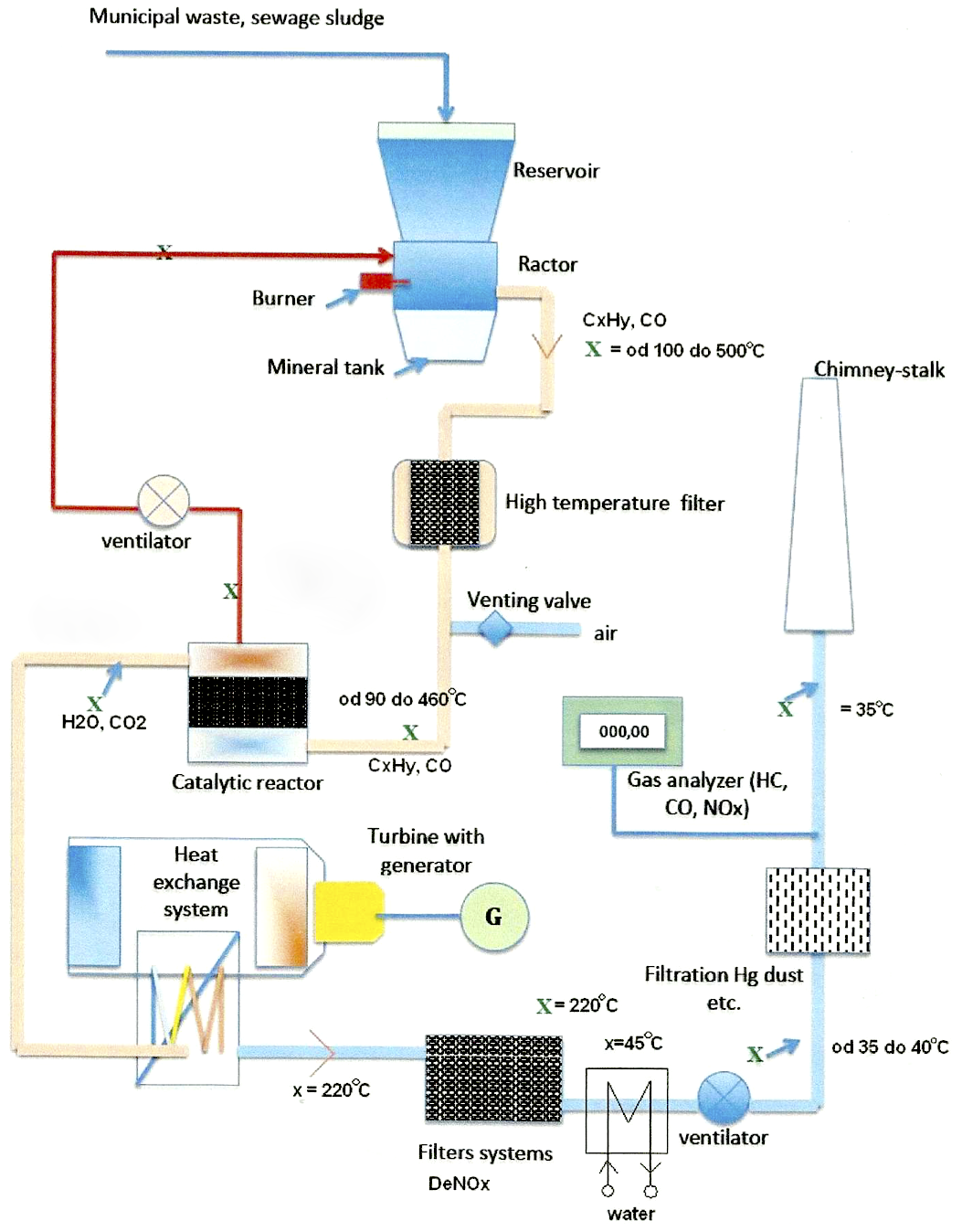
After modification of in-coming waste by separation and griding on required fraction upto 50 mm use to be transported by transport line (belt) into reaction chamber storage bin, from which the reaction chamber is filled by gravitation.
Reaction chamber after opening the gas burners is warmed up on processing temperature approx. 450°C. After chamber is filled by waste the whole humidity upto 200°C temperature will be devaporised and the mineralization, gasification and ulitization process starts. The temperatures are floeating from 500°C to 550°C depending on processed waste sort.
During mineralization process the material is becoming dry and than decay. Carbon will be gasificated on CO2 or on other organic substances which are decayed on catalyzers on CO2 and H2O. the whole carbon will be used while on-going reactions are making the chain of gas hydrocarbons, which are catalyticaly divided on CO2 and H2O and inert material in accordance with the content of organic mass.
Parts of metal and glass from waste are not oxidating in mineralization process because of low temperature of the process and negligable presence of oxygene 3% and from process are out-going without any change.
Catalytic mineralization is the process where from organic materials arise a mineral. Volume of in-coming waste slope down 20-100 multiplied depending on its content. Processed – utilized waste leave from reaction chamber in a form of fine mineral powder and is from the bin, which is situated in the bottom, transported by belt into collecting container.
The mineralization process ended so far and technicla cleanign gas process starts. The temperature will be accomodated by falling on 400°C not damage the catalyzers. After that the reaction gas is transported on high temperature filters, it is floating through and will be cleaned from all minerals and dirt not to damage the catalyzers. It is transported further to catalytic burner where it is oxigenated by air based on that its temperature will increase on 600°C. In reactor the reaction takes place where CxHY and CO is going to be changed into H2O and CO2. From reactor 5% of heated gas will be transported to burning chamber while it will be heated and it is no more necessary to warm it by gas burner. The remaining gas part of 95% goes to heat converter which is working on base of Exchange (air – air, air – water, air – oil). Temperature in heat converter is going down from 600°C on 22°C. From the convereter can the air going out or water steam into turbogenerator which is producing electric energy according to output or is going into catalytic reactor DeNOx where will be reduced NOx on NO2.
Catalyzers are working by optimal temperature. Gas will be decayed on catalyzers are arising CO and CO2 and substances of NOx. Substances of chlorine are deacying from gas which are further processed or will be alkalized (catching the material base on fluid) on sorbets and raising CO2.
After the reaction gas is cleaned up in reserve oxidator. The level of cleaning is 99,9%.
Further the gas is transported into heat converter where its temperature is falling from 220°C on 35°C and from there use to be transported into chimney where is going out in form of water steam CO2 and H2O. In case we are going to catch H2O, which is in volume of 600 liters/ ton of waste that we use to launch just CO2.